
H2S Molecular Geometry Science Education and Tutorials
H2S Molecular Geometry / Shape and Bond Angles (Note: precise bond angle is 92.1 degrees.) Wayne Breslyn 715K subscribers Join Subscribe Subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4.

So far, we’ve used 8 of the H2S Lewis structure’s total 8 outermost valence shell electrons. Two
Geometry of Molecules. Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.

H2S Lewis structure Polar, Molecular geometry, Science education
H2S Molecular and Electron Geometry based on the VSEPR theory, the steric number, Hybridization and expected bond angles.
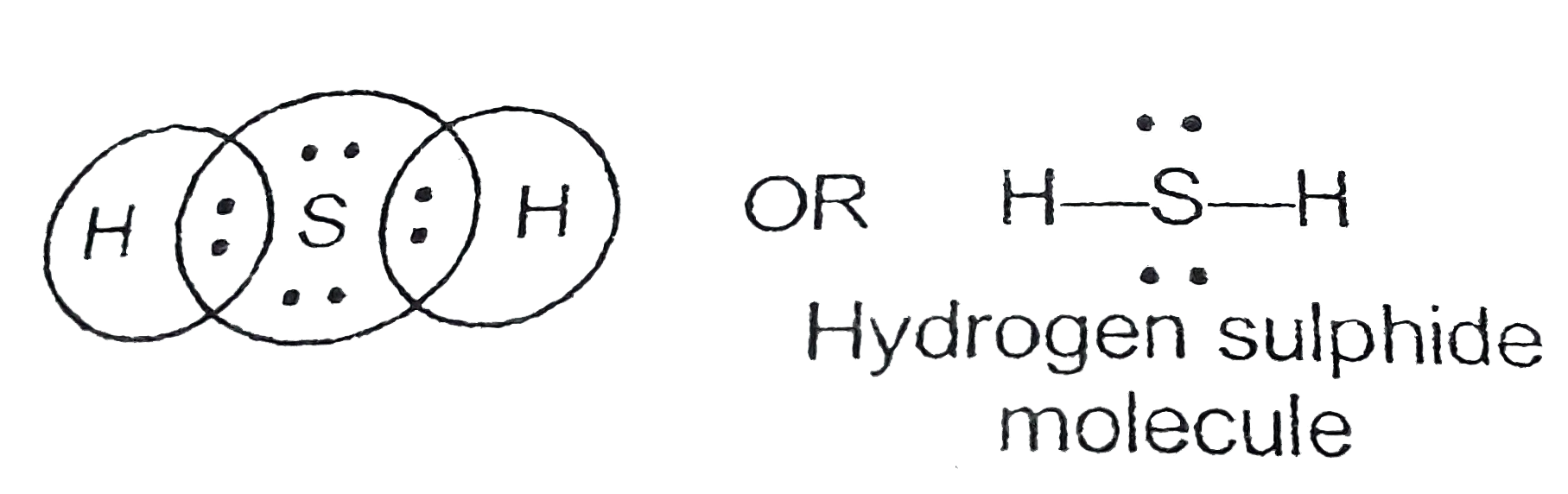
31+ H2S Lewis Structure Pictures Bepe Enthusiastic
Molecular Geometry of Hydrogen Sulfide (H 2 S) Hydrogen sulfide (H 2 S) molecule consists of one sulfur (S) atom and two hydrogen (H) atoms. Hydrogen (H) is located in Group 1, and sulfur (S) is in Group 16 of the periodic table. Hydrogen has one, and sulfur has six valence electrons. The total number of valence electrons in hydrogen sulfide is 8.

H2S (Hydrogen sulfide) Molecular Geometry, Bond Angles YouTube
Step 1: Find out the total number of valence electrons in the molecule. Do take care of +, - signs while calculating. Step 2: Choose a central atom; generally the atom with the highest bonding sites. Step 3: Draw a skeletal structure with single bonds only. Step 4: Fill up the octet of the atoms with the remaining electrons.

H2s hydrogen sulfide molecule Royalty Free Vector Image
An explanation of the molecular geometry for the H2S ion (Hydrogen sulfide) including a description of the H2S bond angles. The electron geometry for the Hyd.
H2S Molecular Geometry H2s Vsepr Term Top Hydrogen sulfide molecular structure
This video explains molecular geometry of H2S molecule by VSEPR theory. According to VSEPR theory, the shape of a covalent molecule depends upon the repulsion between the electron pairs in.
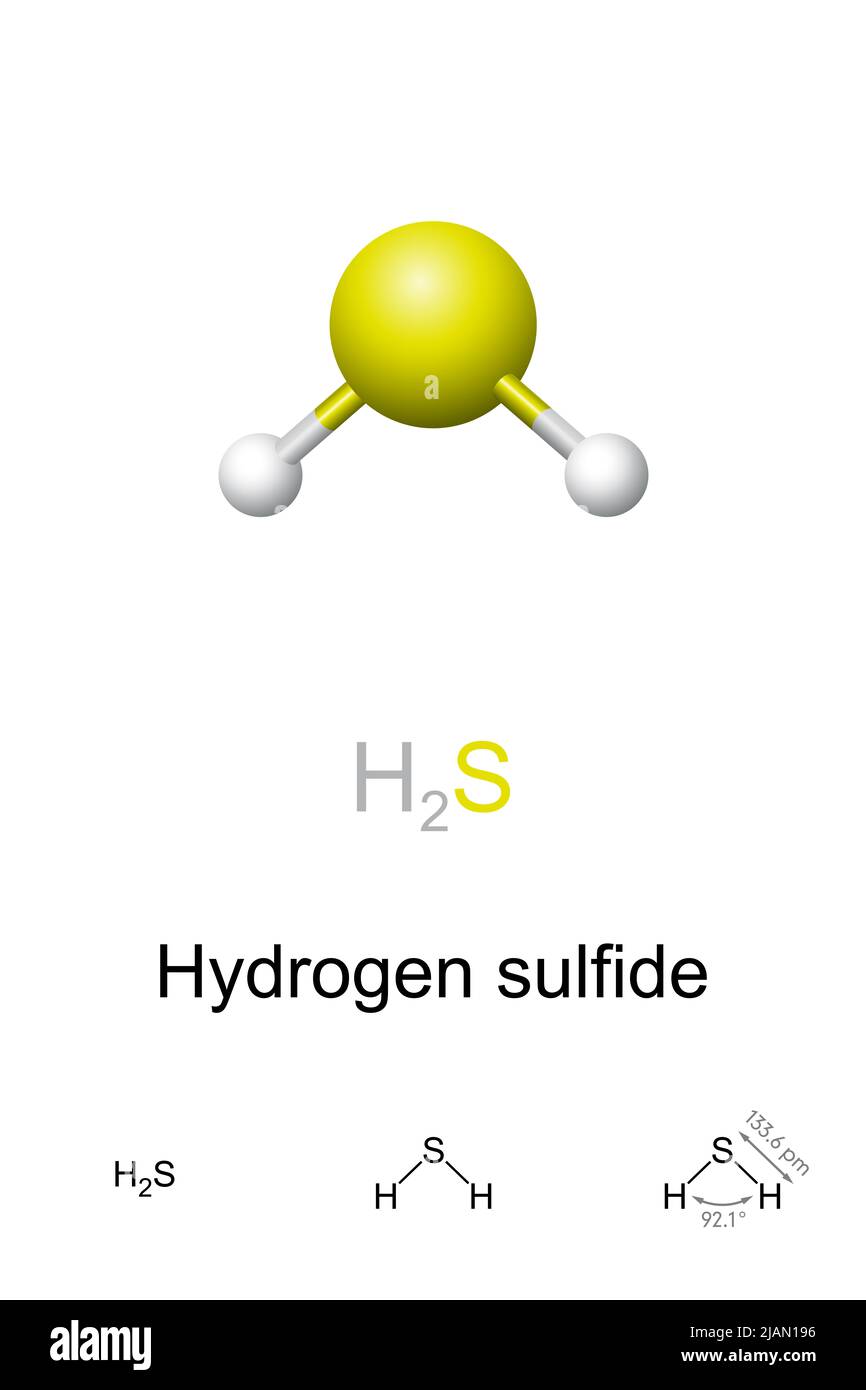
Hydrogen sulfide, ballandstick model, molecular and chemical formula. Chemical compound with
The hydrogen sulfide (H2S) molecule is classified as a polar molecule. The molecule of hydrogen sulfide (with tetrahedral or bent V-shaped molecular geometry) is tilted, the bond angles between sulfur and hydrogen are 92 degrees.

H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar,Octet Rule
H2S is a chemical formula of Hydrogen Sulphide gas. It is highly toxic, poisonous and flammable. In this video, we will look at the molecular structure of an.
H2s molecular geometry singleatila
The total number of electrons around the central atom, S, is eight, which gives four electron pairs. Two of these electron pairs are bonding pairs and two are lone pairs, so the molecular geometry of \(\ce{H2S}\) is bent (Figure \(\PageIndex{6}\)). The bond dipoles cannot cancel one another, so the molecule has a net dipole moment.
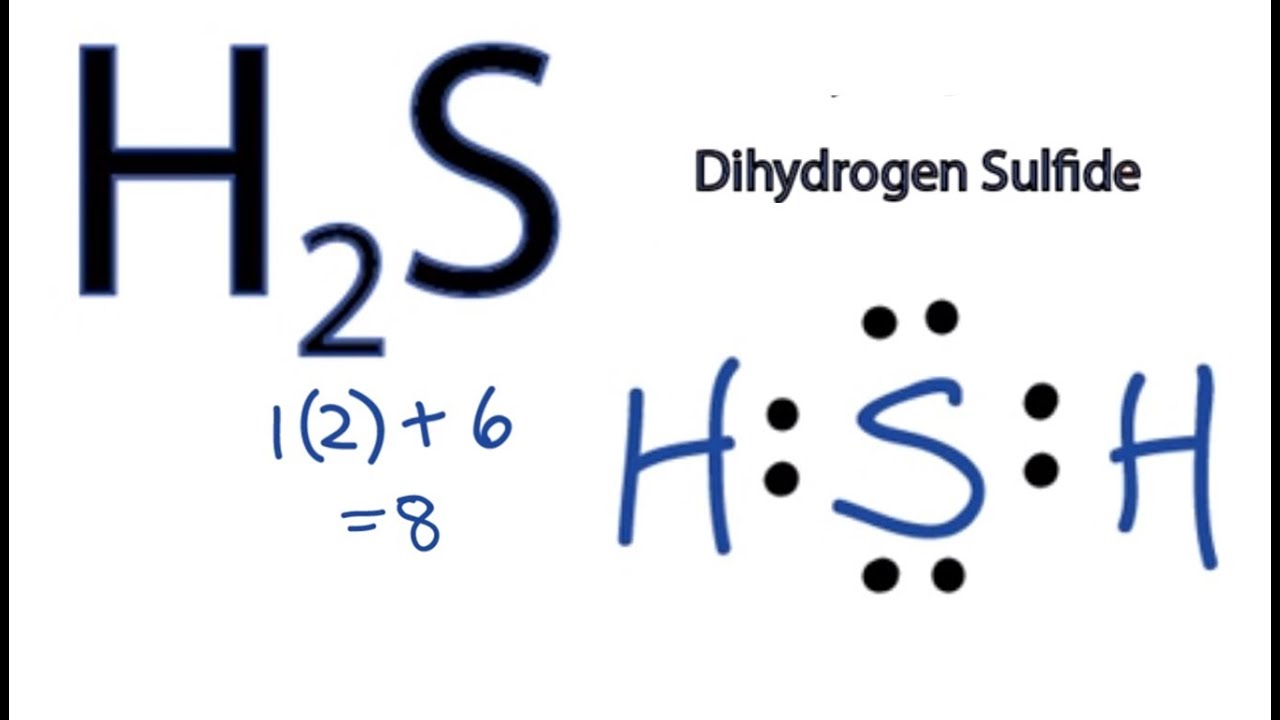
Estructura De Lewis H2s Estudiar
Try the eBay way-getting what you want doesn't have to be a splurge. Browse A molecular! Find the deal you deserve on eBay. Discover discounts from sellers across the globe.
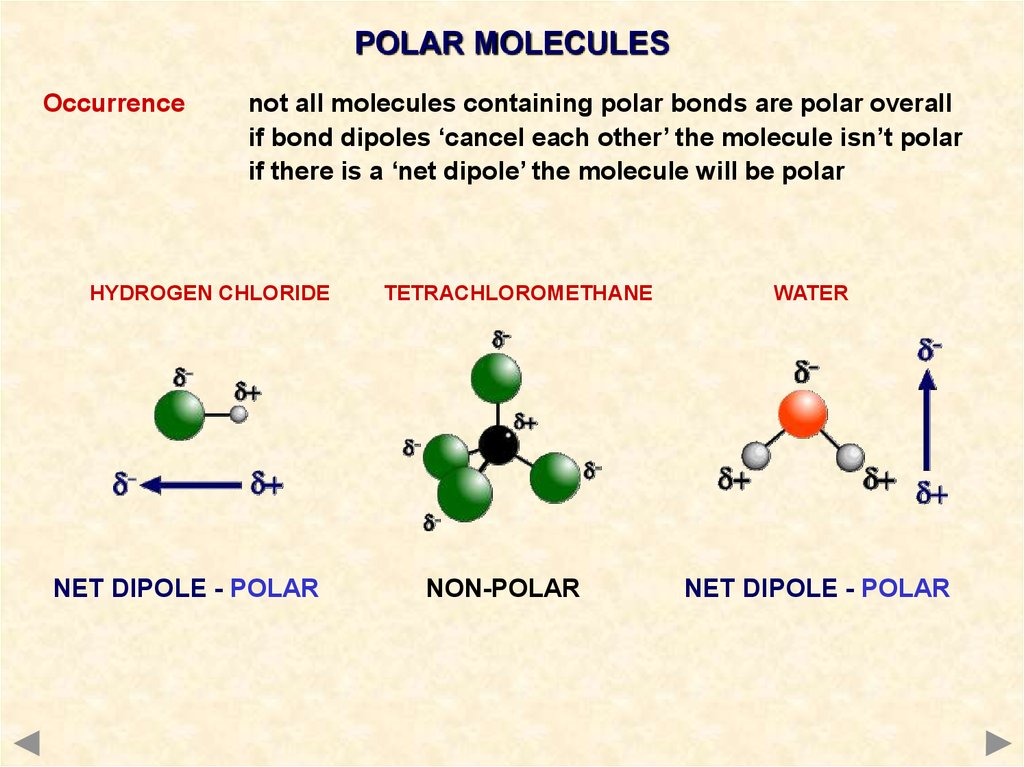
H2s molecular geometry boothulsd
Geometry. To determine if H 2 S (hydrogen sulfide) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. There are 6 + 2 = 8 electrons, and 4 of them are used to make 2 bonds.

H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
Molecular Geometry of H2S A. Determination of the shape of H2S molecule. By considering the arrangement of atoms and lone pairs around the central sulfur atom, one can determine the molecular geometry of H2S. H2S has two bonding pairs and two lone pairs of electrons on the sulfur atom. Due to the repulsion between lone pairs, the molecule.
Hydrogen Sulfide Molecule Photograph by Molekuul/science Photo Library Pixels
The first step is to sketch the molecular geometry of the H2S molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the H2S hybridization, and the third step is to give perfect notation for the H2S molecular geometry.

Hydrogen sulfide H₂S Molecular Geometry Hybridization Molecular Weight Molecular Formula
Written by Priyanka in Science Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H2S. The molecule has two Hydrogen atoms and a single Sulfur atom. H2S is also a precursor for elemental Sulfur.

H2s molecular geometry singleatila
The total number of electrons around the central atom, S, is eight, which gives four electron pairs. Two of these electron pairs are bonding pairs and two are lone pairs, so the molecular geometry of \(\ce{H2S}\) is bent (Figure \(\PageIndex{6}\)). The bond dipoles cannot cancel one another, so the molecule has a net dipole moment.